Study results of Wegovy® (semaglutide) injection 2.4 mg
Wegovy® is the first and only FDA-approved once-weekly GLP-1 RA medication to treat adults with MASH with liver scarring,* along with a reduced calorie diet and increased physical activity.
*Moderate to advanced liver scarring (fibrosis) without cirrhosis.
GLP-1 RA, glucagon-like peptide-1 receptor agonist; MASH, metabolic dysfunction–associated steatohepatitis.
Actor portrayal.
How Wegovy® was studied
Results are from Week 72 of a medical study of adults with MASH with moderate to advanced liver scarring (fibrosis), but not with cirrhosis. 800 adults were included in the analysis. Health care professionals (HCPs) involved in the study were encouraged to optimize treatment for coexisting conditions and provide healthy lifestyle counseling for all participants.
Reduced MASH and no worsening of liver scarring
In the study, significantly more adults taking Wegovy® achieved a reduction in MASH and no worsening of liver scarring† compared with those taking placebo (inactive injection). This means that more patients taking Wegovy® achieved:
- Mild to no inflammation in the liver
- No enlarged liver cells, also called ballooning
- No worsening of liver scarring

†Defined as resolution of steatohepatitis (score of 0-1 for lobular inflammation, 0 for ballooning, and any value for steatosis) and no worsening of liver scarring.
Improved liver scarring and no worsening of MASH
In the study, significantly more adults taking Wegovy® achieved an improvement in liver scarring and no worsening of MASH‡ compared with those taking placebo. This means that more patients taking Wegovy® achieved:
- A decrease in liver scarring by at least one stage in severity
- No worsening of MASH
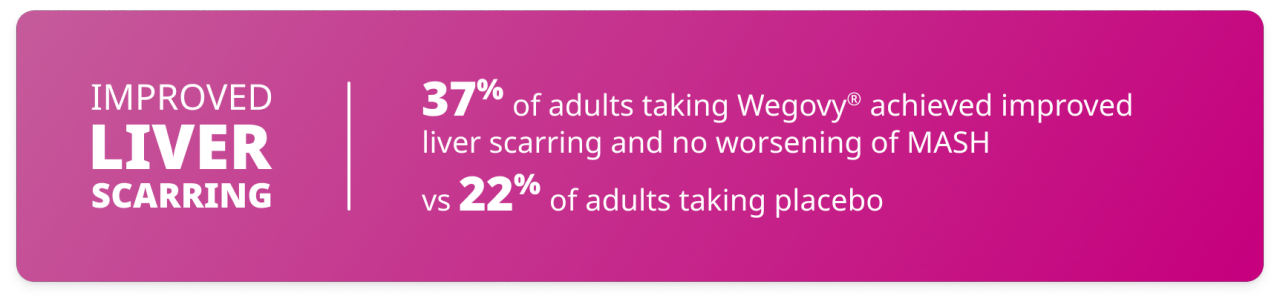
‡Defined as at least one stage improvement in liver fibrosis and no worsening of steatohepatitis (defined as no increase from baseline in score for ballooning, lobular inflammation, or steatosis).
Actor portrayal.
Could Wegovy® help you take on MASH?
If you're living with MASH with liver scarring, you can take action with treatment. Wegovy® can treat MASH with liver scarring, along with the positive changes you are working to make through diet and physical activity.
About the Wegovy® medical study
800 adults with MASH with moderate to advanced liver scarring (fibrosis), but not cirrhosis, were included in the analysis at Week 72.
In the study, many adults with MASH had other related health conditions. About 73% had obesity, 56% had type 2 diabetes, 63% had high blood pressure, and 25% had dyslipidemia.
All adults in the study were randomly assigned to 2 groups. 534 were allocated to the group receiving Wegovy® (semaglutide) injection 2.4 mg once weekly, and 266 were allocated to the group receiving placebo once weekly. Health care professionals involved in the study were encouraged to optimize treatment for coexisting conditions and provide healthy lifestyle counseling for all patients.
To measure results during the study, a liver biopsy (small sample of liver tissue) at 72 weeks was compared to a biopsy taken before the person was treated in the study.
Ask your HCP about other indications for Wegovy®
Talk to your doctor if you have questions and want to know more about the other Wegovy® indications.
Wegovy® is also indicated in combination with a reduced calorie diet and increased physical activity:
- To reduce the risk of major adverse cardiovascular (CV) events (CV death, non-fatal myocardial infarction, or non-fatal stroke) in adults with established CV disease and either obesity or overweight. Learn more here.
- To reduce excess body weight and maintain weight reduction long term in:
- Adults with obesity
- Adults with overweight with at least one weight-related comorbid condition. Learn more here.
Important Safety Information
What is the most important information I should know about Wegovy®?
Wegovy® may cause serious side effects, including:
- Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rodents, Wegovy® and medicines that work like Wegovy® caused thyroid tumors, including thyroid cancer. It is not known if Wegovy® will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people
- Do not use Wegovy® if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
Do not use Wegovy® if:
- you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
- you have had a serious allergic reaction to semaglutide or any of the ingredients in Wegovy®
Before using Wegovy®, tell your healthcare provider if you have any other medical conditions, including if you:
- have or have had problems with your pancreas or kidneys
- have type 2 diabetes and a history of diabetic retinopathy
- have or have had depression, suicidal thoughts, or mental health issues
- are scheduled to have surgery or other procedures that use anesthesia or deep sleepiness (deep sedation)
- are pregnant or plan to become pregnant. Wegovy® may harm your unborn baby. You should stop using Wegovy® 2 months before you plan to become pregnant
- are breastfeeding or plan to breastfeed. It is not known if Wegovy® passes into your breast milk
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Wegovy® may affect the way some medicines work and some medicines may affect the way Wegovy® works. Tell your healthcare provider if you are taking other medicines to treat diabetes, including sulfonylureas or insulin. Wegovy® slows stomach emptying and can affect medicines that need to pass through the stomach quickly.
What are the possible side effects of Wegovy®?
Wegovy® may cause serious side effects, including:
- inflammation of your pancreas (pancreatitis). Stop using Wegovy® and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without nausea or vomiting. Sometimes you may feel the pain from your abdomen to your back
- gallbladder problems. Wegovy® may cause gallbladder problems, including gallstones. Some gallstones may need surgery. Call your healthcare provider if you have symptoms, such as pain in your upper stomach (abdomen), fever, yellowing of the skin or eyes (jaundice), or clay-colored stools
- increased risk of low blood sugar (hypoglycemia), especially those who also take medicines for diabetes such as insulin or sulfonylureas. This can be a serious side effect. Talk to your healthcare provider about how to recognize and treat low blood sugar and check your blood sugar before you start and while you take Wegovy®. Signs and symptoms of low blood sugar may include dizziness or light-headedness, blurred vision, anxiety, irritability or mood changes, sweating, slurred speech, hunger, confusion or drowsiness, shakiness, weakness, headache, fast heartbeat, or feeling jittery
- dehydration leading to kidney problems. Diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems. It is important for you to drink fluids to help reduce your chance of dehydration. Tell your healthcare provider right away if you have nausea, vomiting, or diarrhea that does not go away
- severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Wegovy®. Tell your healthcare provider if you have stomach problems that are severe or will not go away
- serious allergic reactions. Stop using Wegovy® and get medical help right away, if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue, or throat; problems breathing or swallowing; severe rash or itching; fainting or feeling dizzy; or very rapid heartbeat
- change in vision in people with type 2 diabetes. Tell your healthcare provider if you have changes in vision during treatment with Wegovy®
- increased heart rate. Wegovy® can increase your heart rate while you are at rest. Tell your healthcare provider if you feel your heart racing or pounding in your chest and it lasts for several minutes
- depression or thoughts of suicide. You should pay attention to any mental changes, especially sudden changes in your mood, behaviors, thoughts, or feelings. Call your healthcare provider right away if you have any mental changes that are new, worse, or worry you
- food or liquid getting into the lungs during surgery or other procedures that use anesthesia or deep sleepiness (deep sedation). Wegovy® may increase the chance of food getting into your lungs during surgery or other procedures. Tell all your healthcare providers that you are taking Wegovy® before you are scheduled to have surgery or other procedures
The most common side effects of Wegovy® may include: nausea, diarrhea, vomiting, constipation, stomach (abdomen) pain, headache, tiredness (fatigue), upset stomach, dizziness, feeling bloated, belching, low blood sugar in people with type 2 diabetes, gas, stomach flu, heartburn, and runny nose or sore throat.
Please see Prescribing Information and Medication Guide for Wegovy®.
Wegovy® is a prescription medication.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
This site is intended for US patients only.
What is Wegovy®?
WEGOVY® (semaglutide) injection 2.4 mg is an injectable prescription medicine used with a reduced calorie diet and increased physical activity to treat adults with metabolic dysfunction-associated steatohepatitis (MASH) with moderate to advanced liver scarring (fibrosis), but not with cirrhosis of the liver.
This indication is approved based on improvement of MASH and liver scarring (fibrosis). There is an ongoing study to confirm the clinical benefit of Wegovy® in adults with MASH.
Wegovy® contains semaglutide and should not be used with other semaglutide-containing products or other GLP-1 receptor agonist medicines.
It is not known if Wegovy® is safe and effective for use for the treatment of MASH in children under 18 years.
What is Wegovy®?
WEGOVY® (semaglutide) injection 2.4 mg is an injectable prescription medicine used with a reduced calorie diet and increased physical activity to treat adults with metabolic dysfunction-associated steatohepatitis (MASH) with moderate to advanced liver scarring (fibrosis), but not with cirrhosis of the liver.
This indication is approved based on improvement of MASH and liver scarring (fibrosis). There is an ongoing study to confirm the clinical benefit of Wegovy® in adults with MASH.
Wegovy® contains semaglutide and should not be used with other semaglutide-containing products or other GLP-1 receptor agonist medicines.
It is not known if Wegovy® is safe and effective for use for the treatment of MASH in children under 18 years.
Important Safety Information
What is the most important information I should know about Wegovy®?
Wegovy® may cause serious side effects, including:
- Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rodents, Wegovy® and medicines that work like Wegovy® caused thyroid tumors, including thyroid cancer. It is not known if Wegovy® will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people
- Do not use Wegovy® if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
Important Safety Information
What is the most important information I should know about Wegovy®?
Wegovy® may cause serious side effects, including:
- Possible thyroid tumors, including cancer. Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer. In studies with rodents, Wegovy® and medicines that work like Wegovy® caused thyroid tumors, including thyroid cancer. It is not known if Wegovy® will cause thyroid tumors or a type of thyroid cancer called medullary thyroid carcinoma (MTC) in people
- Do not use Wegovy® if you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
Do not use Wegovy® if:
- you or any of your family have ever had a type of thyroid cancer called medullary thyroid carcinoma (MTC) or if you have an endocrine system condition called Multiple Endocrine Neoplasia syndrome type 2 (MEN 2)
- you have had a serious allergic reaction to semaglutide or any of the ingredients in Wegovy®
Before using Wegovy®, tell your healthcare provider if you have any other medical conditions, including if you:
- have or have had problems with your pancreas or kidneys
- have type 2 diabetes and a history of diabetic retinopathy
- have or have had depression, suicidal thoughts, or mental health issues
- are scheduled to have surgery or other procedures that use anesthesia or deep sleepiness (deep sedation)
- are pregnant or plan to become pregnant. Wegovy® may harm your unborn baby. You should stop using Wegovy® 2 months before you plan to become pregnant
- are breastfeeding or plan to breastfeed. It is not known if Wegovy® passes into your breast milk
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Wegovy® may affect the way some medicines work and some medicines may affect the way Wegovy® works. Tell your healthcare provider if you are taking other medicines to treat diabetes, including sulfonylureas or insulin. Wegovy® slows stomach emptying and can affect medicines that need to pass through the stomach quickly.
What are the possible side effects of Wegovy®?
Wegovy® may cause serious side effects, including:
- inflammation of your pancreas (pancreatitis). Stop using Wegovy® and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without nausea or vomiting. Sometimes you may feel the pain from your abdomen to your back
- gallbladder problems. Wegovy® may cause gallbladder problems, including gallstones. Some gallstones may need surgery. Call your healthcare provider if you have symptoms, such as pain in your upper stomach (abdomen), fever, yellowing of the skin or eyes (jaundice), or clay-colored stools
- increased risk of low blood sugar (hypoglycemia), especially those who also take medicines for diabetes such as insulin or sulfonylureas. This can be a serious side effect. Talk to your healthcare provider about how to recognize and treat low blood sugar and check your blood sugar before you start and while you take Wegovy®. Signs and symptoms of low blood sugar may include dizziness or light-headedness, blurred vision, anxiety, irritability or mood changes, sweating, slurred speech, hunger, confusion or drowsiness, shakiness, weakness, headache, fast heartbeat, or feeling jittery
- dehydration leading to kidney problems. Diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems. It is important for you to drink fluids to help reduce your chance of dehydration. Tell your healthcare provider right away if you have nausea, vomiting, or diarrhea that does not go away
- severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use Wegovy®. Tell your healthcare provider if you have stomach problems that are severe or will not go away
- serious allergic reactions. Stop using Wegovy® and get medical help right away, if you have any symptoms of a serious allergic reaction, including swelling of your face, lips, tongue, or throat; problems breathing or swallowing; severe rash or itching; fainting or feeling dizzy; or very rapid heartbeat
- change in vision in people with type 2 diabetes. Tell your healthcare provider if you have changes in vision during treatment with Wegovy®
- increased heart rate. Wegovy® can increase your heart rate while you are at rest. Tell your healthcare provider if you feel your heart racing or pounding in your chest and it lasts for several minutes
- depression or thoughts of suicide. You should pay attention to any mental changes, especially sudden changes in your mood, behaviors, thoughts, or feelings. Call your healthcare provider right away if you have any mental changes that are new, worse, or worry you
- food or liquid getting into the lungs during surgery or other procedures that use anesthesia or deep sleepiness (deep sedation). Wegovy® may increase the chance of food getting into your lungs during surgery or other procedures. Tell all your healthcare providers that you are taking Wegovy® before you are scheduled to have surgery or other procedures
The most common side effects of Wegovy® may include: nausea, diarrhea, vomiting, constipation, stomach (abdomen) pain, headache, tiredness (fatigue), upset stomach, dizziness, feeling bloated, belching, low blood sugar in people with type 2 diabetes, gas, stomach flu, heartburn, and runny nose or sore throat.
Please see Prescribing Information and Medication Guide for Wegovy®.
Wegovy® is a prescription medication.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
This site is intended for US patients only.
What is Wegovy®?
WEGOVY® (semaglutide) injection 2.4 mg is an injectable prescription medicine used with a reduced calorie diet and increased physical activity to treat adults with metabolic dysfunction-associated steatohepatitis (MASH) with moderate to advanced liver scarring (fibrosis), but not with cirrhosis of the liver.
This indication is approved based on improvement of MASH and liver scarring (fibrosis). There is an ongoing study to confirm the clinical benefit of Wegovy® in adults with MASH.
Wegovy® contains semaglutide and should not be used with other semaglutide-containing products or other GLP-1 receptor agonist medicines.
It is not known if Wegovy® is safe and effective for use for the treatment of MASH in children under 18 years.

